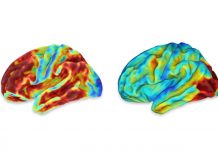
A drug for treating Alzheimer’s disease that the biotechnology company Biogen left for dead in March may get another chance to prove itself.
Biogen, based in Cambridge, Massachusetts, announced on 22 October that it would seek approval from the US Food and Drug Administration for its drug aducanumab to treat early-stage Alzheimer’s disease, for which there is currently no available treatment.
The company had halted development of aducanumab because an early analysis of clinical-trial results suggested that it had no significant effects on clinical symptoms such as memory loss and disorientation.
But Biogen has since evaluated new data from the same studies. These showed that for a subset of patients, high doses of aducanumab given for an extended period significantly slowed down cognitive decline.
Biogen’s share price, which plummeted in March following news…