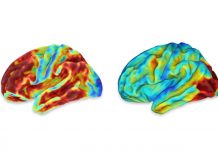
Aducanumab, a controversial Alzheimer’s disease medication with “questionable efficacy,” is under review by the Therapeutic Goods Administration, but authors of a Perspective published today by the Medical Journal of Australia say “science, not desperation” should guide the process.
Aducanumab is a human monoclonal antibody that selectively reacts with amyloid-β, reducing amyloid plaque. The most dominant theory about the development of Alzheimer’s disease (AD) is that the initial causative event is a deposition of amyloid-β.
The U.S. Food and Drug Administration (FDA) approved aducanumab for use in AD despite Phase 3 trials…