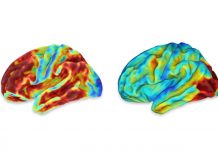
(HealthDay)—Tauvid (flortaucipir F18), a radioactive diagnostic agent, was approved to image tau pathology in patients with cognitive impairment being evaluated for Alzheimer disease, the U.S. Food and Drug Administration announced Thursday.
The drug is indicated for positron emission tomography (PET) imaging to estimate the density and distribution of aggregated tau neurofibrillary tangles (NFTs). Tauvid is delivered via intravenous injection and binds to brain sites associated with the tau protein, which can be identified through PET scan imaging.
The approval was based on data from two clinical studies in which five blinded evaluators interpreted the Tauvid imaging. In the first study, 156 terminally ill patients agreed to…