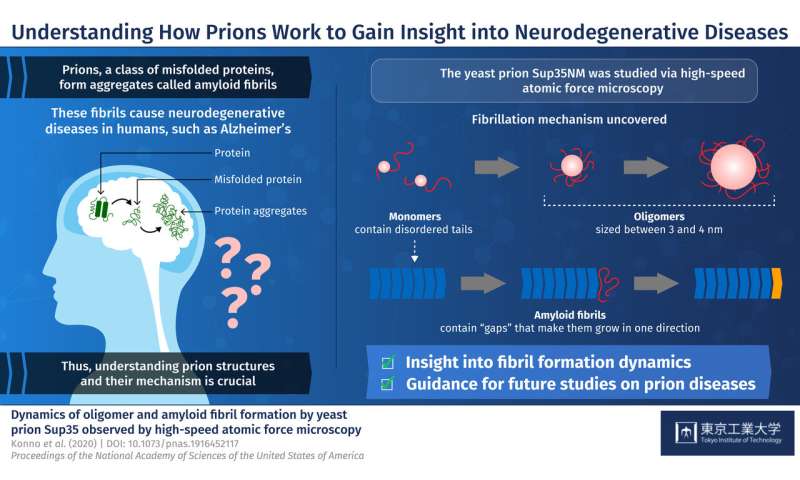
Prions are a class of misfolded proteins that form aggregates called amyloid fibrils. These aggregates are the main culprit behind severe mammalian neurodegenerative diseases like Alzheimer’s. What makes them so deadly is that they are capable of transmitting their erroneous conformation to otherwise healthy proteins, causing an imbalance in cellular function. Currently, there are no effective treatments for fatal prion diseases, mainly because studying mammalian prions is challenging. Thus, scientists have turned to studying prions in less complex organisms like yeast,…



























